4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis
Related Articles: 4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to 4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: 4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis
- 2 Introduction
- 3 4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis
- 3.1 Understanding DMAP’s Structure and Properties
- 3.2 DMAP’s Role in Acyl Transfer Reactions
- 3.3 Applications of DMAP in Organic Synthesis
- 3.4 Benefits of Using DMAP as a Catalyst
- 3.5 FAQs regarding DMAP
- 3.6 Tips for Using DMAP in Organic Synthesis
- 3.7 Conclusion
- 4 Closure
4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis
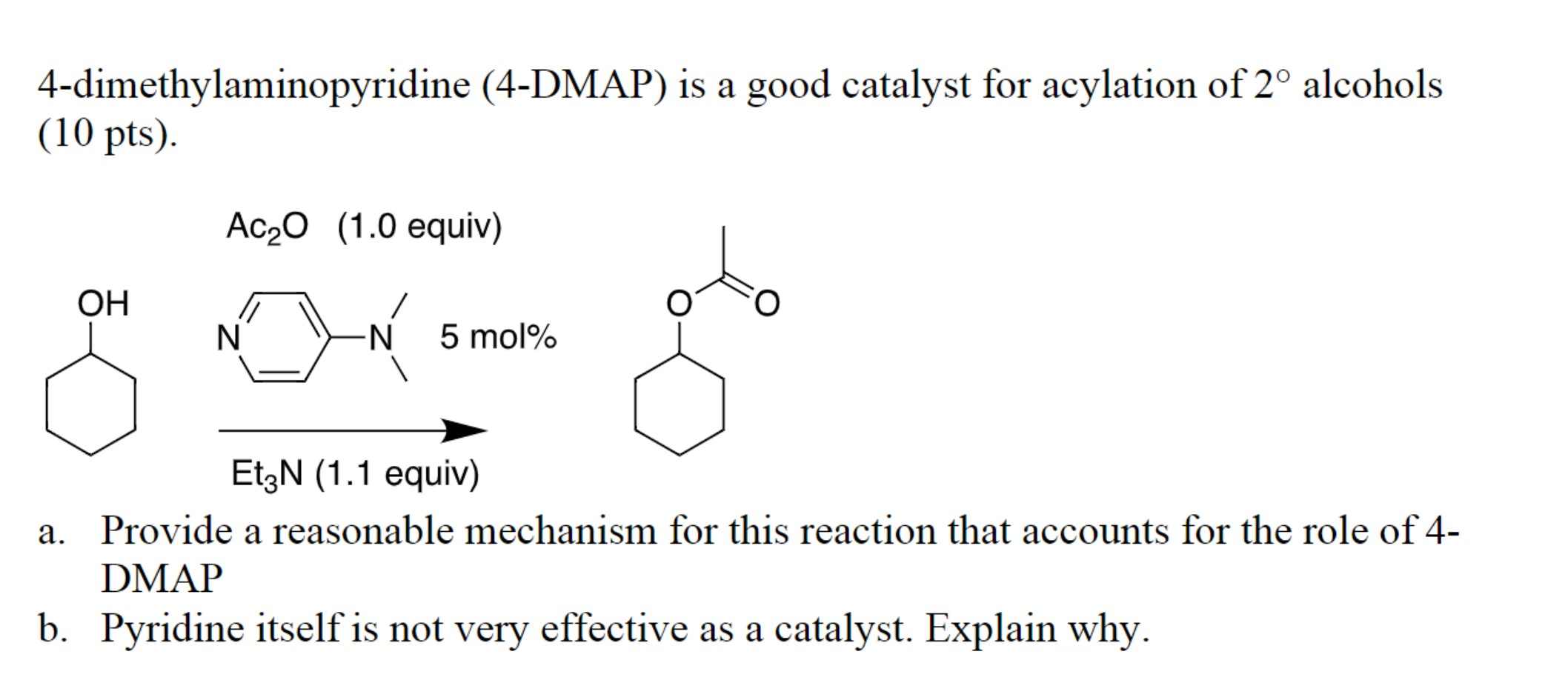
4-Dimethylaminopyridine (DMAP), a heterocyclic organic compound with the CAS number [1122-58-3], holds a prominent position in the field of organic synthesis. This versatile reagent, known for its potent catalytic activity, plays a crucial role in a wide array of chemical transformations, particularly in the realm of acyl transfer reactions.
Understanding DMAP’s Structure and Properties
DMAP’s structure features a pyridine ring, a six-membered aromatic heterocycle containing a nitrogen atom, with two methyl groups attached at the 4-position. This specific arrangement of atoms imparts several key properties to DMAP:
- Strong Electron-Donating Ability: The two methyl groups on the nitrogen atom significantly increase the electron density of the pyridine ring, making DMAP a powerful electron donor.
- Nucleophilic Character: This enhanced electron density translates into a pronounced nucleophilic character, making DMAP readily capable of interacting with electrophilic species.
- Acyl Transfer Catalysis: DMAP’s nucleophilic nature and the presence of the pyridine ring, which can readily accommodate an acyl group, make it a highly effective catalyst for acyl transfer reactions.
DMAP’s Role in Acyl Transfer Reactions
Acyl transfer reactions involve the transfer of an acyl group (R-C=O) from one molecule to another. This type of reaction is fundamental in organic synthesis, forming the basis for the formation of esters, amides, and other important functional groups.
DMAP’s catalytic activity in acyl transfer reactions stems from its ability to form a highly reactive intermediate, a DMAP-acylpyridinium ion. This intermediate is generated by the reaction of DMAP with an acylating agent, such as an acid chloride or anhydride.
The DMAP-acylpyridinium ion is a highly electrophilic species that readily reacts with a nucleophile, such as an alcohol or amine, to form the desired product. The reaction proceeds through a two-step mechanism:
- Nucleophilic Attack: DMAP attacks the carbonyl carbon of the acylating agent, forming a tetrahedral intermediate.
- Elimination: The tetrahedral intermediate undergoes elimination of a leaving group, resulting in the formation of the DMAP-acylpyridinium ion.
This mechanism effectively facilitates the transfer of the acyl group from the acylating agent to the nucleophile, significantly accelerating the reaction rate.
Applications of DMAP in Organic Synthesis
DMAP’s catalytic properties make it a valuable tool in a wide range of synthetic applications:
- Esterification: DMAP efficiently catalyzes the formation of esters from carboxylic acids and alcohols. This reaction is widely employed in the synthesis of pharmaceuticals, polymers, and other important organic compounds.
- Amide Formation: DMAP is an excellent catalyst for the formation of amides from carboxylic acids and amines. This reaction is essential in the synthesis of peptides, proteins, and other biologically active molecules.
- Acylation of Alcohols and Amines: DMAP effectively catalyzes the acylation of alcohols and amines, leading to the formation of various valuable organic compounds.
- Ring-Opening Reactions: DMAP has been successfully employed in ring-opening reactions of cyclic carbonates and lactones.
- Silylation Reactions: DMAP facilitates the silylation of alcohols and amines, leading to the formation of protected derivatives.
Benefits of Using DMAP as a Catalyst
- High Efficiency: DMAP exhibits high catalytic activity, significantly accelerating the rate of acyl transfer reactions.
- Mild Reaction Conditions: Reactions catalyzed by DMAP often proceed under mild conditions, minimizing the risk of side reactions and degradation of sensitive substrates.
- Versatility: DMAP is compatible with a wide range of substrates and reaction conditions, making it a versatile reagent for diverse synthetic applications.
- Ease of Handling: DMAP is a commercially available reagent, readily accessible and easy to handle.
FAQs regarding DMAP
Q: What is the mechanism of DMAP catalysis in acyl transfer reactions?
A: DMAP acts as a nucleophile, attacking the carbonyl carbon of the acylating agent to form a reactive intermediate, the DMAP-acylpyridinium ion. This intermediate then readily reacts with a nucleophile to form the desired product.
Q: What are the advantages of using DMAP over other catalysts in acyl transfer reactions?
A: DMAP offers several advantages, including high efficiency, mild reaction conditions, versatility, and ease of handling.
Q: How does the presence of the methyl groups on the nitrogen atom affect DMAP’s catalytic activity?
A: The methyl groups enhance the electron density of the pyridine ring, making DMAP a stronger electron donor and a more effective nucleophile, which contributes to its catalytic activity.
Q: What are some common applications of DMAP in organic synthesis?
A: DMAP is widely used in esterification, amide formation, acylation of alcohols and amines, ring-opening reactions, and silylation reactions.
Q: Are there any safety concerns associated with DMAP?
A: DMAP is generally considered safe to handle, but it is important to follow proper safety protocols, including wearing appropriate personal protective equipment and working in a well-ventilated area.
Tips for Using DMAP in Organic Synthesis
- Purity: Use high-quality, anhydrous DMAP to ensure optimal catalytic activity.
- Stoichiometry: Use the appropriate stoichiometry of DMAP and other reagents to ensure efficient reaction progress.
- Solvent Selection: Choose a suitable solvent that is compatible with the reaction and the reagents.
- Temperature Control: Monitor the reaction temperature to avoid unwanted side reactions or decomposition of the reagents.
- Work-up: Use appropriate work-up procedures to isolate the desired product and remove any residual DMAP.
Conclusion
DMAP, with its CAS number [1122-58-3], has established itself as an indispensable tool in the arsenal of organic chemists. Its remarkable catalytic activity, particularly in acyl transfer reactions, has significantly advanced the development of efficient and selective synthetic methods. DMAP’s versatility, ease of handling, and compatibility with a wide range of substrates make it a valuable reagent for both academic and industrial research. As research continues, DMAP’s role in organic synthesis is poised to expand further, leading to the discovery of new and innovative synthetic methodologies.








Closure
Thus, we hope this article has provided valuable insights into 4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis. We hope you find this article informative and beneficial. See you in our next article!