A Comprehensive Exploration of 4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis
Related Articles: A Comprehensive Exploration of 4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to A Comprehensive Exploration of 4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
A Comprehensive Exploration of 4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis

4-Dimethylaminopyridine, commonly known as DMAP, stands as a powerful and versatile catalyst in organic synthesis, renowned for its ability to accelerate and improve a wide array of reactions. Its unique properties, stemming from its electron-rich aromatic structure, have made it an indispensable tool for chemists across diverse fields, from pharmaceutical development to materials science.
Understanding the Structure and Properties of DMAP
DMAP’s chemical structure is relatively simple, yet it holds the key to its exceptional catalytic activity. It comprises a pyridine ring, a six-membered heterocyclic system containing nitrogen, with two methyl groups attached to the nitrogen atom in the para position. This structural arrangement bestows DMAP with several crucial properties:
-
Electron-Rich Nature: The presence of the dimethylamino group (-N(CH3)2) directly attached to the aromatic ring makes DMAP highly electron-rich. This electron density is readily available for donation, enabling DMAP to act as a strong nucleophile.
-
Strong Base: DMAP’s electron-rich character also makes it a relatively strong base, capable of deprotonating acidic compounds. This basicity plays a crucial role in many of its catalytic applications.
-
Excellent Leaving Group: The dimethylamino group, being a strong electron donor, stabilizes the positive charge that develops on the nitrogen atom upon leaving. This makes DMAP an excellent leaving group, facilitating the formation of reactive intermediates in various reactions.
DMAP’s Role as a Catalyst in Organic Synthesis
DMAP’s exceptional properties make it an ideal catalyst for a wide range of reactions, including:
-
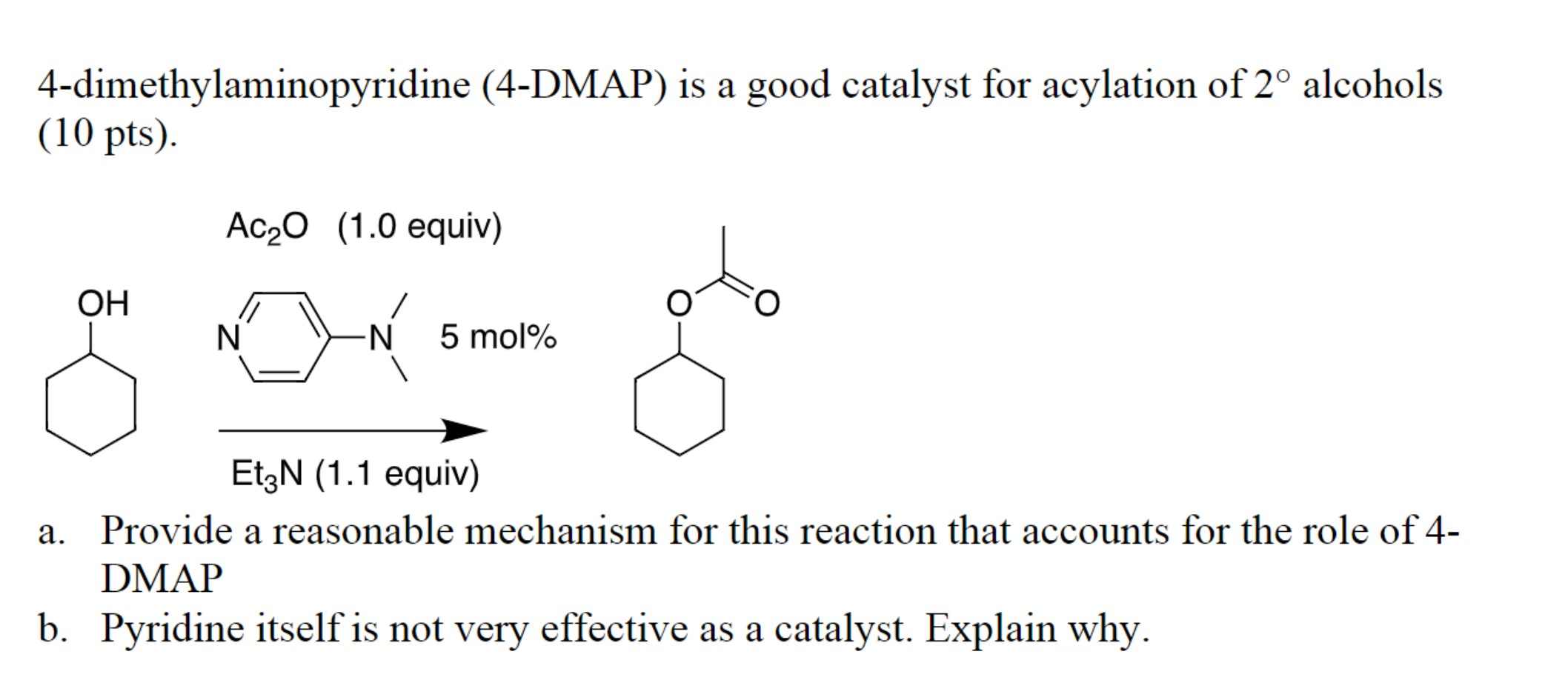
Acylation Reactions: DMAP’s ability to accelerate acylation reactions, particularly those involving hindered substrates, is perhaps its most well-known application. It acts as a nucleophilic catalyst, forming an activated intermediate with the acylating agent, which then readily reacts with the nucleophile. This mechanism allows for the efficient formation of esters, amides, and other acyl derivatives, even under mild conditions.
-
Esterification Reactions: DMAP’s catalytic prowess extends to esterification reactions, where it facilitates the formation of esters from carboxylic acids and alcohols. This application is particularly valuable in the synthesis of complex molecules, where traditional esterification methods might prove inefficient or require harsh conditions.
-
Ring-Opening Reactions: DMAP’s ability to activate electrophilic species makes it a valuable catalyst in ring-opening reactions. It can facilitate the opening of cyclic compounds, such as lactones and lactams, by promoting the attack of a nucleophile on the electrophilic center.
-
Carbonyl Addition Reactions: DMAP can also catalyze carbonyl addition reactions, where nucleophiles add to carbonyl compounds. This application is particularly useful in the synthesis of alcohols, amines, and other compounds containing a carbon-carbon bond formed via nucleophilic addition.
-
Other Applications: DMAP’s versatility extends beyond these common applications. It finds utility in various other transformations, including:
- Dehydration Reactions: DMAP can facilitate the removal of water from alcohols, promoting the formation of alkenes.
- Silylation Reactions: DMAP can catalyze the reaction of alcohols with silyl halides, leading to the formation of silyl ethers.
- Alkylation Reactions: DMAP can enhance the reactivity of alkyl halides in alkylation reactions, facilitating the formation of new carbon-carbon bonds.
Benefits of Using DMAP as a Catalyst
The use of DMAP as a catalyst offers several advantages in organic synthesis:
-
Increased Reaction Rates: DMAP’s catalytic activity significantly accelerates reactions, reducing reaction times and improving efficiency.
-
Mild Reaction Conditions: DMAP often allows for reactions to be carried out under milder conditions, minimizing the formation of unwanted byproducts and improving product purity.
-
Enhanced Selectivity: DMAP can enhance the selectivity of reactions, favoring the formation of the desired product over undesired isomers or side products.
-
Improved Yields: DMAP can lead to higher yields of the desired product, maximizing the efficiency of the synthesis process.
Factors Influencing DMAP’s Catalytic Activity
Several factors can influence DMAP’s catalytic activity, including:
-
Nature of the Substrate: The structure and reactivity of the substrate can influence DMAP’s effectiveness. For example, DMAP is particularly effective for reactions involving hindered substrates, where other catalysts might struggle.
-
Nature of the Acylating Agent: The reactivity of the acylating agent can also affect DMAP’s catalytic activity. More reactive acylating agents, such as acid chlorides, tend to react faster in the presence of DMAP.
-
Solvent Effects: The choice of solvent can significantly impact DMAP’s catalytic activity. Polar aprotic solvents, such as dichloromethane and THF, are often preferred for DMAP-catalyzed reactions.
-
Temperature and Concentration: Temperature and concentration can also affect DMAP’s activity. Higher temperatures generally lead to faster reaction rates, while increasing the concentration of DMAP can also enhance its catalytic effect.
FAQs Regarding DMAP
1. What are the safety precautions associated with DMAP?
DMAP is a corrosive substance and can cause skin and eye irritation. It is essential to handle DMAP with appropriate personal protective equipment, such as gloves, goggles, and a lab coat. Additionally, it should be stored in a well-ventilated area away from heat and oxidizing agents.
2. How is DMAP typically used in a reaction?
DMAP is typically used in catalytic amounts, ranging from 1 to 10 mol%. It is added to the reaction mixture before or during the addition of the acylating agent. The reaction is usually carried out in a polar aprotic solvent, such as dichloromethane or THF.
3. What are some common alternatives to DMAP?
Several alternatives to DMAP are available, each with its own advantages and disadvantages. Some common alternatives include:
- Triethylamine (Et3N): A less expensive and more readily available base, but often less effective than DMAP.
- Pyridine: A classic catalyst, but less reactive than DMAP and can lead to the formation of unwanted side products.
- N-Methylmorpholine (NMM): A milder base and more selective catalyst than DMAP, but may be less effective for certain reactions.
4. How can I determine the optimal conditions for a DMAP-catalyzed reaction?
Optimizing a DMAP-catalyzed reaction involves carefully considering several factors, including:
- Stoichiometry of reactants: The ratio of reactants can significantly impact the reaction outcome.
- Choice of solvent: Selecting the appropriate solvent can influence the reaction rate and selectivity.
- Temperature: Varying the reaction temperature can affect the rate and yield of the reaction.
- Catalyst loading: The amount of DMAP used can influence the reaction rate and efficiency.
5. What are some examples of DMAP-catalyzed reactions in real-world applications?
DMAP finds application in various fields, including:
- Pharmaceutical Synthesis: DMAP is widely used in the synthesis of pharmaceuticals, such as antibiotics and antivirals.
- Materials Science: DMAP is used in the synthesis of polymers, resins, and other materials with specific properties.
- Agricultural Chemistry: DMAP is used in the synthesis of pesticides and herbicides.
- Fine Chemical Synthesis: DMAP is used in the synthesis of fragrances, dyes, and other specialty chemicals.
Tips for Using DMAP in Organic Synthesis
- Start with a small amount of DMAP: It is generally advisable to start with a low catalyst loading (1-5 mol%) and gradually increase the amount if necessary.
- Choose the appropriate solvent: Select a polar aprotic solvent, such as dichloromethane or THF, that is compatible with the reactants and catalyst.
- Monitor the reaction progress: Track the reaction progress using analytical techniques, such as TLC or NMR, to ensure the reaction is proceeding as expected.
- Consider alternative catalysts: If DMAP proves ineffective or leads to unwanted side products, explore alternative catalysts that might be more suitable for the specific reaction.
Conclusion
DMAP stands as a powerful and versatile catalyst in organic synthesis, offering numerous advantages over traditional methods. Its ability to accelerate reactions, enhance selectivity, and improve yields makes it a valuable tool for chemists across diverse fields. Understanding the factors influencing DMAP’s catalytic activity and applying the appropriate techniques can significantly enhance the efficiency and success of organic synthesis reactions. As research continues to explore new applications of DMAP, its role in advancing chemical science is poised to expand even further.



![[PDF] 4-Dimethylaminopyridine as a catalyst in heroin synthesis. Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/bd2da3cfe7d11eafd93ccc26ca55c536e20d40f0/3-Figure2-1.png)


Closure
Thus, we hope this article has provided valuable insights into A Comprehensive Exploration of 4-Dimethylaminopyridine (DMAP): A Versatile Catalyst in Organic Synthesis. We hope you find this article informative and beneficial. See you in our next article!