The Power of Dimethylaminopyridine: A Catalyst for Enhanced Chemical Reactions
Related Articles: The Power of Dimethylaminopyridine: A Catalyst for Enhanced Chemical Reactions
Introduction
With great pleasure, we will explore the intriguing topic related to The Power of Dimethylaminopyridine: A Catalyst for Enhanced Chemical Reactions. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Power of Dimethylaminopyridine: A Catalyst for Enhanced Chemical Reactions
![[PDF] 4-Dimethylaminopyridine as a catalyst in heroin synthesis. Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/bd2da3cfe7d11eafd93ccc26ca55c536e20d40f0/3-Figure2-1.png)
Dimethylaminopyridine (DMAP) is a versatile organic compound that has revolutionized the field of organic chemistry by acting as a highly efficient catalyst for a wide range of reactions. Its unique properties have made it an indispensable tool for researchers and synthetic chemists, enabling them to achieve transformations that were previously challenging or impossible. This article delves into the intricacies of DMAP’s catalytic activity, exploring its mechanism, applications, and the profound impact it has had on various chemical disciplines.
Understanding DMAP’s Catalytic Prowess
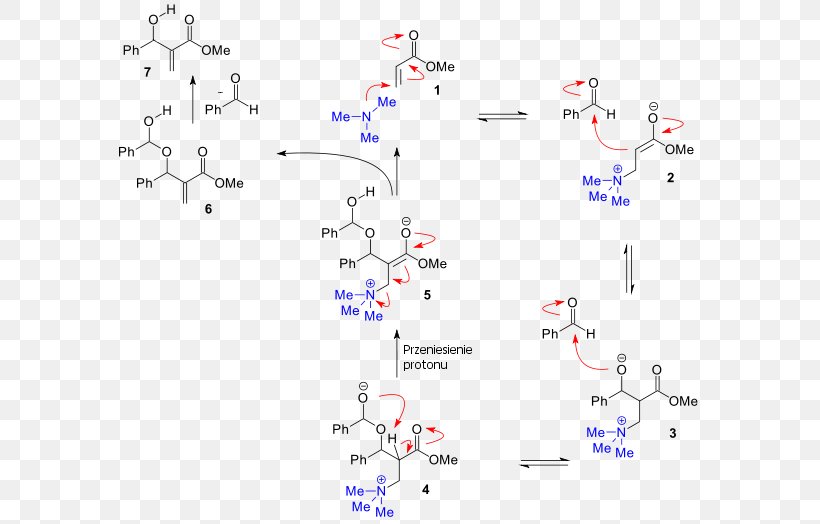
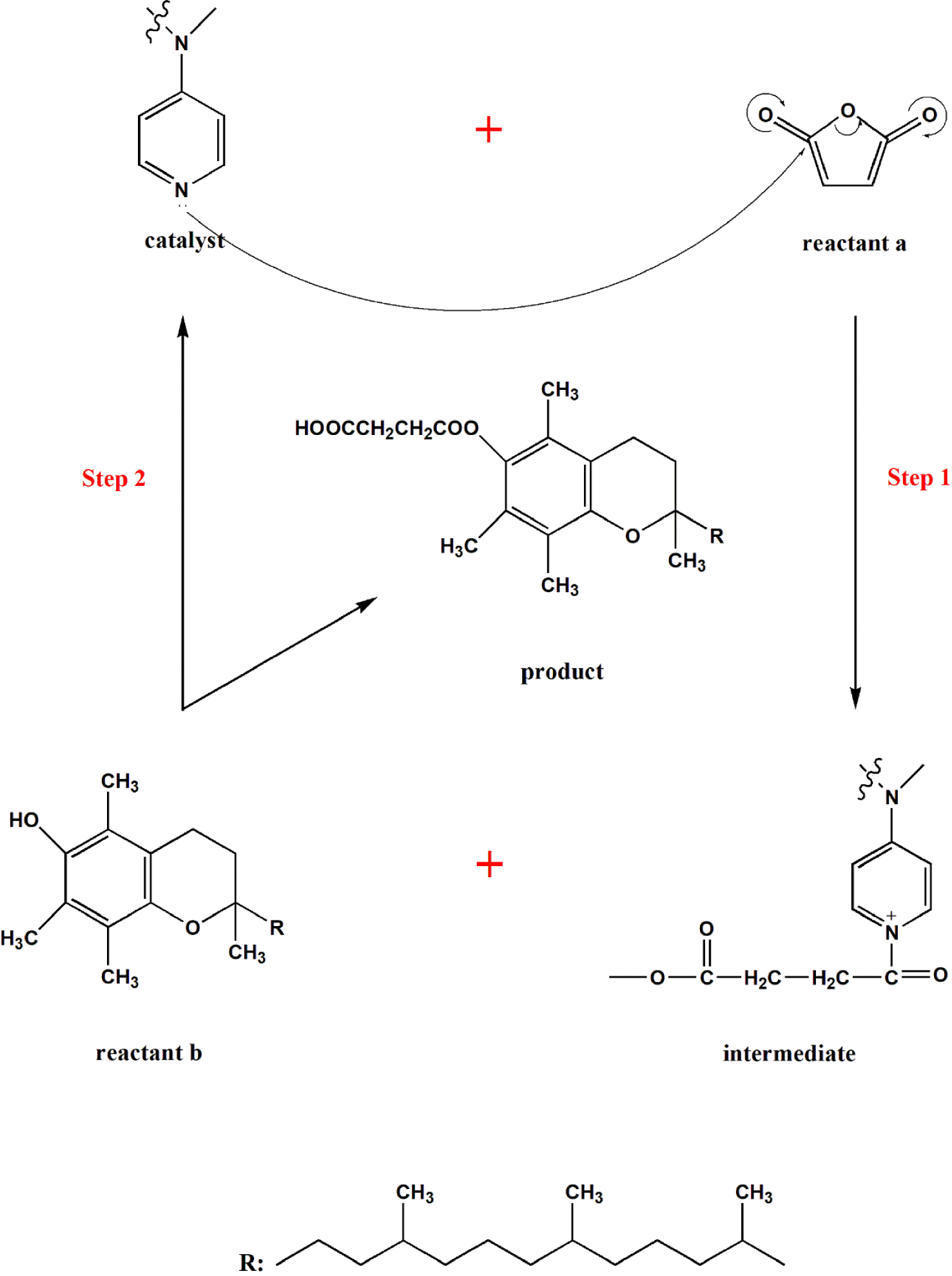
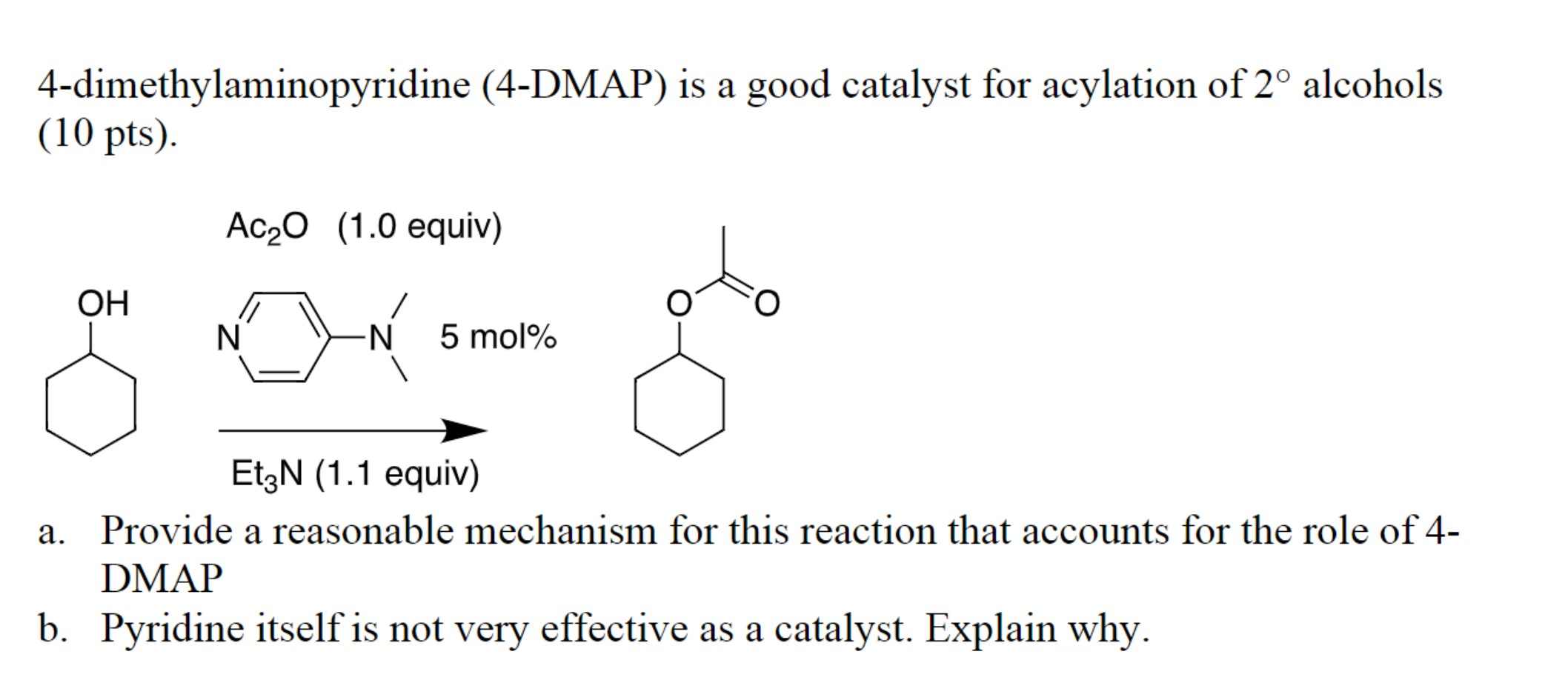
DMAP’s catalytic prowess stems from its ability to accelerate chemical reactions by lowering the activation energy required for the reaction to proceed. This occurs through a series of steps involving the formation of a reactive intermediate, which facilitates the reaction pathway. The key to DMAP’s effectiveness lies in its structure, which features a pyridine ring with a strongly electron-donating dimethylamino group attached at the para position.
Mechanism of DMAP Catalysis
The catalytic mechanism of DMAP typically involves the following steps:
- Nucleophilic Attack: DMAP, with its electron-rich nitrogen atom, acts as a nucleophile and attacks the electrophilic center of the substrate, forming an intermediate. This intermediate is stabilized by the electron-donating dimethylamino group, enhancing its reactivity.
- Leaving Group Departure: The intermediate facilitates the departure of the leaving group from the substrate, leading to the formation of a reactive carbocation.
- Nucleophilic Attack on the Carbocation: The carbocation, now highly electrophilic, is susceptible to attack by a nucleophile, leading to the formation of the desired product.
Applications of DMAP in Organic Synthesis
DMAP’s versatility extends to a wide range of applications in organic synthesis, making it a cornerstone for numerous synthetic strategies. Some of its notable applications include:
- Acylation Reactions: DMAP is an exceptional catalyst for acylation reactions, where an acyl group is transferred from one molecule to another. This is particularly useful in the synthesis of esters, amides, and other carbonyl compounds.
- Alkylation Reactions: DMAP facilitates the alkylation of various substrates, including alcohols, amines, and phenols. This process involves the transfer of an alkyl group from one molecule to another, leading to the formation of new carbon-carbon bonds.
- Ring-Opening Reactions: DMAP effectively catalyzes ring-opening reactions, where a cyclic molecule undergoes a reaction that breaks its ring structure. This is particularly useful in the synthesis of acyclic compounds from cyclic precursors.
- Silylation Reactions: DMAP plays a crucial role in silylation reactions, where a silyl group is introduced into a molecule. This process is widely used in protecting functional groups and in the synthesis of silicon-containing compounds.
- Polymerization Reactions: DMAP has also found applications in polymerization reactions, where it acts as a catalyst for the formation of long-chain polymers. This is particularly relevant in the synthesis of polyesters, polyamides, and other synthetic polymers.
DMAP in Pharmaceutical and Material Science
DMAP’s applications extend beyond organic synthesis, finding its way into various other chemical disciplines. In pharmaceutical chemistry, DMAP is employed in the synthesis of complex drug molecules, aiding in the formation of essential functional groups and chiral centers. In material science, DMAP is used in the preparation of advanced materials, including polymers, composites, and nanomaterials, contributing to their enhanced properties and functionalities.
Benefits of Using DMAP as a Catalyst
DMAP offers several advantages over other catalysts, making it a preferred choice in many synthetic endeavors:
- High Efficiency: DMAP is highly efficient, catalyzing reactions at low concentrations and achieving high yields. This translates to reduced reaction times and improved overall productivity.
- Mild Reaction Conditions: DMAP often operates under mild reaction conditions, minimizing the need for harsh temperatures or pressures. This is particularly beneficial for sensitive substrates and delicate functional groups.
- Selectivity: DMAP exhibits high selectivity in reactions, leading to the formation of specific products with minimal side reactions. This is crucial for obtaining desired products with high purity.
- Versatility: DMAP is compatible with a wide range of substrates and reaction conditions, making it a versatile tool for diverse synthetic applications.
FAQs on DMAP
1. What are the safety precautions when handling DMAP?
DMAP is a corrosive and irritant substance. It should be handled with care in a well-ventilated area, wearing appropriate personal protective equipment such as gloves, goggles, and lab coats.
2. How can I purify DMAP?
DMAP can be purified by recrystallization from a suitable solvent like diethyl ether or toluene.
3. How can I determine the optimal amount of DMAP to use in a reaction?
The optimal amount of DMAP depends on the specific reaction and substrate. It is often determined through experimentation by varying the amount of DMAP and monitoring the reaction progress.
4. Are there any alternatives to DMAP as a catalyst?
Yes, there are several alternatives to DMAP, including other pyridine derivatives like 4-pyrrolidinopyridine (PPY) and 4-(dimethylamino)pyridine N-oxide (DMAPO). The choice of catalyst depends on the specific reaction and desired outcome.
5. How can I monitor the progress of a DMAP-catalyzed reaction?
The progress of a DMAP-catalyzed reaction can be monitored using various analytical techniques like thin-layer chromatography (TLC), gas chromatography (GC), or nuclear magnetic resonance (NMR) spectroscopy.
Tips for Using DMAP Effectively
- Use High-Quality DMAP: Ensure that the DMAP used is of high purity to minimize side reactions and ensure optimal catalytic activity.
- Optimize Reaction Conditions: Fine-tune the reaction conditions, such as temperature, solvent, and stoichiometry, to achieve the best results.
- Monitor Reaction Progress: Regularly monitor the reaction progress using suitable analytical techniques to ensure the reaction proceeds as expected.
- Handle with Care: Always handle DMAP with appropriate safety precautions, wearing protective gear and working in a well-ventilated area.
Conclusion
DMAP has emerged as a powerful catalyst in organic chemistry, revolutionizing synthetic strategies and enabling the efficient synthesis of a wide range of compounds. Its unique properties, including its high efficiency, mild reaction conditions, and versatility, have made it an indispensable tool for researchers and synthetic chemists alike. As our understanding of DMAP’s catalytic activity continues to grow, its applications in various fields, including pharmaceuticals, materials science, and beyond, are expected to expand further, contributing to advancements in diverse scientific disciplines.




Closure
Thus, we hope this article has provided valuable insights into The Power of Dimethylaminopyridine: A Catalyst for Enhanced Chemical Reactions. We appreciate your attention to our article. See you in our next article!